Articles & Product Documents, Clinical Journals
Duration, complications, stress, and pain of open ovariohysterectomy versus a simple method of laparoscopic-assisted ovariohysterectomy in dogs
If you need to print this PDF or save for later
click below to download your copy.
Duration, complications, stress, and pain of open ovariohysterectomy versus a simple method of laparoscopic-assisted ovariohysterectomy in dogs
Chad M. Devitt, DVM, MS, DACVS; Ray E. Cox, DVM; Jim J. Hailey, DVM
Objective—To describe a simple method of laparoscopic-assisted ovariohysterectomy (LAOHE) and compare duration of surgery, complications, measures of surgical stress, and postoperative pain with open ovariohysterectomy (OHE) in dogs.
Design—Randomized, prospective clinical trial.
Animals—20 healthy sexually intact female dogs weighing > 10 kg (22 lb).
Procedures—Dogs were randomly allocated to receive conventional OHE or LAOHE. Intraoperative complications, anesthetic complications, total anesthesia time, and total surgery time were recorded. Serum cortisol and glucose concentrations, temperature, heart rate, and respiratory rate were measured preoperatively and 1, 2, 4, 6, 12, and 24 hours postoperatively. Pain scores were assigned by a nonblinded observer at 1, 2, 4, 6, 12, and 24 hours postoperatively. Duration of surgery, pain scores, objective measures of surgical stress, anesthetic complications, and surgical complications were compared between OHE and LAOHE.
Results—Age, weight, PCV, and duration of surgery did not differ between treatment groups. Nine of 10 dogs in the OHE group required additional pain medication on the basis of pain scores, whereas none of the dogs in the LAOHE group did. Blood glucose concentrations were significantly increased from preoperative concentrations in the OHE group at 1, 2, 4, and 6 hours postoperatively and at 1 hour postoperatively in the LAOHE group. Cortisol concentrations were significantly increased at 1 and 2 hours postoperatively in the OHE group.
Conclusions and Clinical Relevance—LAOHE caused less pain and surgical stress than OHE and may be more appropriate for an outpatient setting. (J Am Vet Med Assoc 2005;227:921–927)
Ovariohysterectomy (OHE) is a common surgical procedure performed in general practice for which the benefits and complications have been described extensively.¹⁻⁸ Laparoscopic methods of OHE are feasible in dogs.⁹⁻¹² However, laparoscopic procedures have not received widespread attention from veterinarians, in part because of the difficulty in mastering laparoscopic techniques, the complexity of the veterinary equipments, and the duration of laparoscopic procedures, compared with open procedures.
Reduced pain associated with laparoscopic OHE has been reported.¹⁰ However, more rigorous comparisons of laparoscopic versus open OHE have yet to establish a clear advantage.ᵃ Some would argue that the benefits of a laparoscopic procedure, compared with a mini-laparotomy as used for OHE, are negligible.¹²⁻¹⁵ Furthermore, prolonged duration of surgery makes laparoscopic procedures less attractive in the typical practice setting.¹⁰
Laparoscopic-assisted procedures maintain minimally invasive attributes of laparoscopic surgery while allowing complex procedures to be performed more efficiently by use of extracorporeal maneuvers. Laparoscopic-assisted procedures have minimal impact, similar to an entirely laparoscopic approach.¹⁶ Laparoscopic-assisted techniques in veterinary medicine such as laparoscopic-assisted gastropexy have reduced the complexity of a minimally invasive gastropexy.¹⁷ This procedure now is routinely used in many practice settings. Similarly, a simple technique for laparoscopic-assisted OHE (LAOHE) was developed. The technique is performed via 2 midline portals, with an operative laparoscope, bipolar cautery, and a specially designed table to permit rotation of the patient from dorsal recumbency to left or right lateral recumbency.
We hypothesized that LAOHE would cause less pain than conventional open OHE, whereas duration of surgery, anesthetic variables, and complications would be similar. The purpose of the study reported here was to compare the duration of surgery, duration of anesthesia, anesthetic complications, surgical complications, and signs of postoperative pain of LAOHE versus open OHE in dogs in a randomized, prospective clinical trial.
Materials and Methods
The institutional animal care and use committee of the Veterinary Referral Center of Colorado approved this protocol. Twenty healthy sexually intact female dogs that weighed > 10 kg (22 lb) and were scheduled for adoption through local animal shelters were randomly allocated to either OHE or LAOHE groups. Owner consent was obtained for dogs that were placed with adopting owners. Consent from the animal shelter director was obtained for all others that were not adopted at the time of the study. Physical evaluation and preoperative CBC and serum biochemical profile were performed and found to be within reference ranges in all dogs.
Twenty-four hours prior to surgery, a 16-gauge through-the-needle catheterᵇ was placed aseptically into the external jugular vein to facilitate and minimize stress associated with blood sample acquisition. If a jugular site was not available, a saphenous vein was used. Serum glucose and cortisol concentrations were measured at baseline after a 24-hour period of acclimation following catheter placement prior to premedication for anesthesia (preoperative) and 1, 2, 4, 6, 12, and 24 hours after extubation. A 10-mL sample of blood was aspirated from the catheter. Serum glucose concentration was measured with a glucometerᶜ immediately after sample acquisition and reported in milligrams per deciliter. Packed cell volume was measured in a standard fashion. A 5-mL aliquot of blood was dispensed into a serum separator tube, clot formation was allowed to occur, and the sample was immediately centrifuged. Following centrifugation, serum was harvested immediately, stored at –80o C in labeled Eppendorf tubes, and evaluated for cortisol concentration at the end of the study by a commercial laboratory via solid-phase radioimmunoassay.ᵈ
Anesthesia—Dogs were preanesthetized with glycopyrrolate (0.01 mg/kg [0.0045 mg/lb], SC), morphine (0.2 mg/kg [0.09 mg/lb], SC), and acepromazine (0.03 mg/kg [0.01 mg/lb], SC). General anesthesia was induced with diazepam (0.2 mg/kg, IV) and propofol (3 mg/kg [1.3 mg/lb], IV) and maintained with isoflurane via endotracheal intubation. Bupivicaine (2 mg/kg [0.9 mg/lb], SC) was infused into the surgical site (OHE) or divided between portal sites (LAOHE) prior to incision. Systolic, diastolic, and mean arterial pressures were measured via arterial catheterization of the dorsal pedal artery or the distal radial artery. Continuous ECG; systolic, diastolic, and mean arterial pressures; heart and respiratory rates; end-tidal (ET) CO2; ET isoflurane; and pulse oximetry SpO2 were monitored and recorded during the duration of the anesthesia. Mechanical ventilation was provided as deemed necessary during anesthesia (PETCO2 > 55 mm Hg).
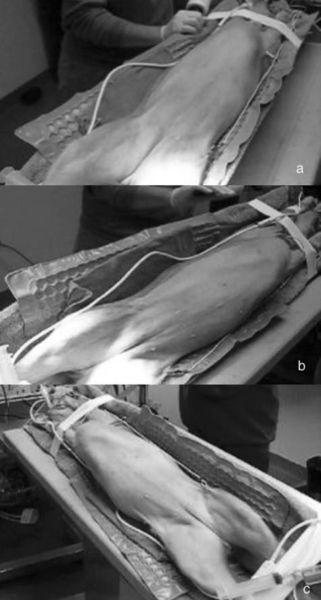
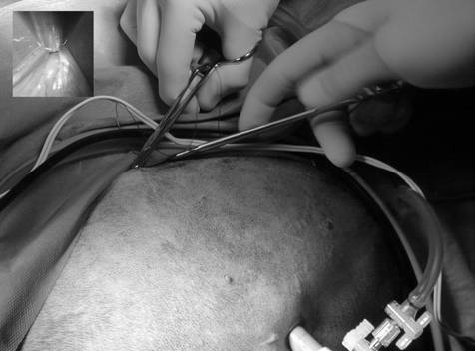
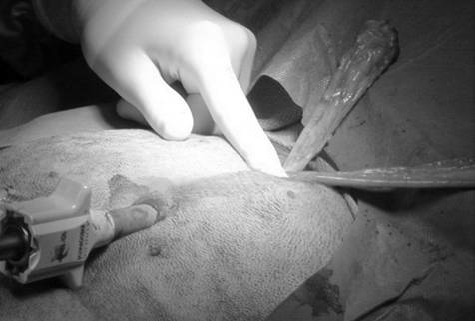
Surgical procedures—All surgeries were performed by 1 surgeon (CMD). Dogs randomly allocated into the OHE group had the procedure performed as described,⁹ with minor modification of the incision size to encompass the middle third of the umbilicopubic distance, rather than the described half of the umbilicopubic distance, to more closely replicate OHEs performed in general practice. Dogs in the LAOHE group were placed in a surgical table (essentially a wooden-hinged V-trough) designed to facilitate rotation of the patient from dorsal recumbency to right or left lateral recumbency while maintaining an aseptic surgical field (Figure 1). While in dorsal recumbency, pneumoperitoneum was attained in a standard fashion (10 to 13 mm Hg) with CO2, a Verres needle, and a mechanical insufflator.ᵉ A 12-mm cannula positioned at the level of the umbilicus was used to insert an 11-mm operative laparoscope with a 6 X 114-mm operating channel (Figure 2).ᶠ Retraction of the spleen and intestines was facilitated by rotation of the patient to right lateral recumbency, which allowed vision of the abdominal viscera and identification of the left ovariouterine complex. The left ovary was grasped and brought to the body wall, which allowed percutaneous advancement of a transabdominal suspension suture (1.0 polydioxanone) with a CT-1 (large taper) needleᵍ to maintain exposure of the ovarian pedicle (Figure 3). With the lights diverted from the surgical field, transillumination of the region was evident. The needle was advanced through the body wall, becoming visible laparoscopically. The needle was directed through the ovariouterine complex, encircling the tissue, and directed out the abdominal wall; the suture was tied, effectively maintaining exposure of the ovarian vasculature.
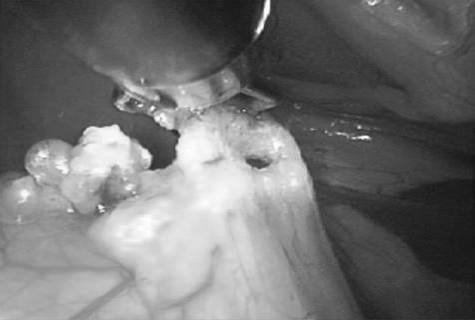
In multiparous animals, additional transabdominal suspension sutures were placed as needed to retract the uterine horn and facilitate exposure of the ovarian pedicle. The suspensory ligament and ovarian vasculature were progressively cauterized and divided with a multifunction bipolar grasping forcepsh (Figure 4). The right ovarian pedicle was identified, exposure was maintained with a transabdominal suspension suture, and the pedicle was progressively cauterized and divided in a similar fashion. Retraction of the intestines and pancreas was facilitated by rotation of the patient to left lateral recumbency (Figure 1). After division of both ovarian pedicles, the patient was returned to dorsal recumbency. A 5- or 12-mm cannula was inserted via direct viewing at the caudal portion of the midline approximately 4 to 5 cm cranial to the pubis (caudal portal). Endosurgical grasping forceps were introduced into the caudal midline portal, and the right horn of the uterus and associated ovary were grasped (Figure 5). The transabdominal suspension suture was released, and the right ovary and associated uterine horn were exteriorized through the abdominal wall via the caudal portal (Figure 6). The caudal portal was enlarged as necessary to allow ease of exteriorization of the ovary. The left ovary and associated uterine horn were exteriorized in a similar fashion. The body of the uterus was ligated, transfixed, and divided in a standard fashion. The ligated uterine stump was reintroduced into the peritoneal cavity through the caudal portal and inspected with the laparoscope for hemostasis and entrapment of regional tissues. The portal sites were closed in 2 layers with monofilament absorbable suture and nylon. Surgical time of OHE was recorded from the initiation of the skin incision to the final skin suture. Surgical time of LAOHE was recorded from the initiation of the pneumoperitoneum to the final skin suture. Total anesthesia time for both groups was from time of induction and intubation to time the vaporizer was set at 0% at the end of the procedure. After surgery, recovery occurred in an isolated environment and blood samples were obtained at 1, 2, 4, 6, 12, and 24 hours after extubation. Morphine was administered after surgery (0.2 mg/kg, SC) to all dogs prior to extubation. Pain scores were assigned on the basis of increase from baseline of heart rate, respiratory rate, mean arterial pressure, behavior, and response to wound palpation at 1, 2, 4, 6, 12, and 24 hours after extubation by 1 of 2 nonblinded technicians (Table 1).¹⁸ Patients with scores > 6 were given additional morphine (0.2 mg/kg, SC).

with bipolar cautery inserted through the 6-mm operating channel of the laparoscope (b).

the uterine horn near the ovary and exteriorize the uterine horn and ovary out of a caudal midline portal in a dog.
Statistical analysis—All statistical analyses were performed by use of statistical software.i Mean and SD values were calculated. Age, weight, surgical time, and total anesthesia time were compared between treatment groups with an unpaired t test. Two-way ANOVA for repeated measures was used to compare the effects of treatment group and time on pain scores. The proportion of patients that required mechanical ventilation during anesthesia and additional morphine was compared between treatment groups with the Fisher exact test, and relative risk was calculated. One-way ANOVA for repeated measures was used to compare differences within treatment groups for blood glucose and cortisol concentrations obtained at various times. If a significant effect was detected, the Dunnett multiple comparison test was used to compare preoperative values with values obtained at each subsequent measurement point. For all comparisons, significance was set at α = 0.05.
| Criteria | Pain Score |
|---|---|
| Heart rate | |
| < 10% | 0 |
| 11% to 30% | 1 |
| 31% to 50% | 2 |
| > 50% | 3 |
| Respiratory rate | |
| < 10% | 0 |
| 11% to 30% | 1 |
| 31% to 50% | 2 |
| > 50% | 3 |
| Crying | |
| Not crying | 0 |
| Crying, responsive | 1 |
| Crying, unresponsive | 2 |
| Mean arterial pressure | |
| < 10% | 0 |
| 11% to 30% | 1 |
| 31% to 50% | 2 |
| > 50% | 3 |
| Movement | |
| None | 0 |
| Position changes | 1 |
| Thrashing | 2 |
| Agitation | |
| Asleep or calm | 0 |
| Mild | 1 |
| Moderate | 2 |
| Hysterical | 3 |
| Surgical site palpation | |
| No response | 0 |
| Turns head | 1 |
| Evades | 2 |
| Tries to bite | 3 |
increase from baseline of heart rate, respiratory rate, and mean
arterial pressure; behavior; and response to wound palpation.
Patients with additive scores ≥ 6 were given additional morphine.
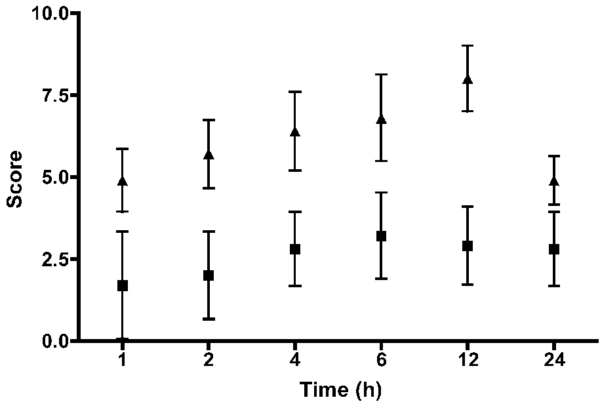
Results
Mean ± SD age was 1.5 ± 0.93 years and 1.45 ± 0.78 years for the OHE and LAOHE groups, respectively (P = 0.444). Mean ± SD weight was 22.1 ± 5.0 kg (48.6 ± 11.0 lb) and 22.0 ± 5.6 kg (48.4 ± 12.3 lb) for the LAOHE and OHE groups, respectively (P = 0.488). Mean ± SD duration of surgery for LAOHE was 20.8 ± 4.0 minutes, and mean duration of surgery for OHE was 18.6 ± 3.9 minutes (P = 0.133). Mean ± SD duration of anesthesia for LAOHE was 46.3 ± 10.0 minutes, and mean duration of anesthesia for OHE was 44.0 ± 9.6 minutes (P = 0.303).
One dog in the OHE group required treatment for hypotension (mean arterial pressure, < 60 mm Hg) with volume expansion (10 mL/kg [4.5 mL/lb], IV bolus) and ephedrine (0.1 mg/kg [0.05 mg/lb], IV). Six dogs (3 in the OHE group and 3 in the LAOHE group) required mechanical ventilation to maintain ETCO2 < 55 mm Hg. During anesthesia, subjective assessment revealed inadequate depth of anesthesia during insufflation of the abdominal cavity with CO2 in 3 of 10 patients in the LAOHE group. Similarly, signs of inadequate depth of anesthesia were detected during digital breakdown of the ovarian pedicle in 9 of 10 patients in the OHE group. Signs of inadequate depth of anesthesia were not detected in any patients during bipolar cautery division of the pedicles.
Surgical complications were not encountered in any dogs in the LAOHE group. In 1 dog in the OHE group, the ovarian pedicle was torn during digital breakdown of the suspensory ligament, which required extension of the incision to locate the bleeding vessel and provide hemostasis. Packed cell volume was not significantly different between or within groups at any time point (P = 0.856).
Pain scores were higher at all points for the OHE group, compared with the LAOHE group (P = 0.001; Figure 7). Nine of 10 dogs in the OHE group required additional pain medication on the basis of pain scores, whereas none in the LAOHE group did (P = 0.001; relative risk, 10.0 [95% confidence interval, 1.6 to 64.2]). Measurements of surgical stress were tabulated (Table 2). Blood glucose concentrations were significantly increased from preoperative concentrations in the OHE group at 1, 2, 4, and 6 hours and in the LAOHE group at 1 hour. Cortisol concentrations were significantly increased from preoperative concentrations at 1 and 2 hours in the OHE group. Cortisol concentrations were not significantly increased in the LAOHE group.
| - | - | - | Time (h) after extubation | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | Group | Preoperative | 1 | 2 | 4 | 6 | 12 | 24 |
| Glucose | LAOHE | 85 ± 14.5 | 95 ± 22.5* | 91 ± 15 | 89 ± 13.4 | 86 ± 12.3 | 85 ± 11.6 | 83 ± 10.4 |
| (mg/dL) | OHE | 73 ± 6.8 | 98 ± 15.8* | 89 ± 8.8* | 85 ± 10.5* | 83 ± 8.5* | 81 ± 7.5 | 83 ± 13.4 |
| Cortisol | LAOHE | 3.4 ± 2.1 | 6.3 ± 4.8 | 4.6 ± 4.5 | 4.2 ± 4.8 | 3.1 ± 2.4 | 2.4 ± 1.9 | 2.1 ± 1.3 |
| (µg/dL) | OHE | 3.1 ± 1.9 | 8.8 ± 3.6* | 6.6 ± 4.2* | 5.2 ± 2.3 | 3.1 ± 2.3 | 2.9 ± 1.9 | 2.2 ± 1.4 |
Discussion
Laparoscopic-assisted ovariohysterectomy as described provided rapid exposure of the ovary and uterus by changing position from dorsal recumbency to lateral recumbency with the use of a specially designed table. Although the table was not necessary to change position, it was useful in maintaining an aseptic surgical field by facilitating rotation from right to left lateral recumbency. Head-down or Trendelenburg positioning was not used. Previously described⁹⁻¹² laparoscopic neutering techniques use 3 or more ports to allow passage of endosurgical graspers or retractors to locate and maintain exposure of the ovariouterine complex during ligation and transection of the ovarian pedicle. Those techniques are inherently time-consuming and can be frustrating if surgical assistance is provided by someone inexperienced in laparoscopic surgery. The use of transabdominal suspension sutures to maintain exposure of the ovarian pedicle eliminated the need for additional portals and a surgical assistant to maintain exposure.²⁰ Additionally, exteriorizing of the uterine horns through the caudal portal and performing extracorporeal ligation and division of the uterine body further simplify the procedure.
Surgical complications were not encountered in the LAOHE group; however, this technique is not without potential complications. Improper use of cautery and transabdominal suspension sutures can bring the structures being transected too close to the abdominal wall, increasing the likelihood of collateral thermal injury, particularly in the hands of an inexperienced laparoscopic surgeon. Strict adherence to proper technique and use of bipolar cautery are important to prevent collateral injury. For example, after grasping the tissue and prior to application of the cautery mode, the tissue must be retracted away from the abdominal wall and other adjacent structures to prevent collateral thermal injury. The complication of subcutaneous migration of CO2 did not occur in the LAOHE group. This was likely because of the method used to create the portal (ie, the closed method), the minimal distension pressure (13 mm Hg), and the short duration of the procedure. Use of an open method to create a portal can increase the risk of this complication if the skin incision is smaller than the body wall incision.19 Additionally, increased distention pressures approaching 15 mm Hg can increase risk. In another veterinary study,⁹ subcutaneous accumulation of CO2 has been reported. In our clinical experience, this is not associated with substantial discomfort and it typically resolves spontaneously without treatment. Careful monitoring and cessation of the laparoscopic procedure are required if subcutaneous emphysema leads to pneumomediastinum or pneumothorax.
The durations of LAOHE and OHE (20.8 vs 18.6 minutes, respectively) were not significantly different. Our study was designed to detect > 15% difference in duration of the procedures with a power of 80%. This difference was chosen as a conservative clinically relevant difference. In a recent comparison10 of laparoscopic OHE versus OHE, the duration of conventional OHE was found to be significantly shorter than laparoscopic OHE (69 vs 129 minutes, respectively). The authors of that study suggest that with experience, consistently performing a laparoscopic OHE in < 1 hour is realistic. Laparoscopic neutering is routinely performed in Europe. In a recent report11 of a large number of dogs that received laparoscopic ovariectomy, the duration of surgery and complications with use of monopolar or bipolar cautery were compared. Duration of surgery was reduced by the use of bipolar cautery to a mean surgical time of 41 minutes. The duration of our method of LAOHE compared favorably with other reported methods of laparoscopic neutering.
Techniques of laparoscopic neutering performed with a harmonic scalpel, vascular clips, or endosurgical suturing methods to secure the ovarian pedicle and the uterine body have been described.⁹,¹⁰ Although an effective and efficient endosurgical tool, a harmonic scalpel is expensive to acquire and use. Bipolar electrocoagulation is a safe, less expensive alternative to the harmonic scalpel. Its use has been reported¹¹,²¹ in horses and dogs for cauterization and transection of the ovarian pedicle. The bipolar forceps used in the dogs of the present study were designed to have multiple functions including grasping, cauterization, and transection of vascular tissue. As such, cumbersome instrument exchanges can be avoided, which further simplifies complex maneuvers and minimizes the number of ports used for the procedure.
Evaluation of postoperative signs of pain is inherently subjective. Postoperative composite pain scores were used to provide a simple method to determine the need for additional pain medication. Mean composite pain scores were significantly lower at all points postoperatively for the LAOHE group, compared with the OHE group. Nine of 10 patients in the OHE group required additional morphine, whereas none of the dogs in the LAOHE group required additional morphine. Relative risk of requiring additional postoperatively administered pain medication in the OHE group was 10 times greater than in the LAOHE group. Unfortunately, no method of pain evaluation is perfect. We used a scoring system used most recently by Walsh et al¹⁸ and used in pediatric human studies²² and veterinary studies.²³ The system is based on increases of easily measured physiologic variables (heart rate, respiratory rate, and mean arterial pressure) and categories of behavioral responses. At each time point, physiologic variables are measured without patient interaction and compared with baseline values. Behavioral responses are categoric and leave little opportunity for introduction of subjective biases. Baseline data were obtained in a standard fashion in all dogs prior to randomization into groups. The advantage of this system is that it is easily applied by technical staff with minimal subjective analysis and training. One disadvantage of this system is the difficulty of applying this method of pain evaluation in clinical practice in other than elective procedures. Other systems, such as a visual analogue scale, would have been more difficult to use to obtain information for the purposes of our study. Visual analogue scale scores are based on the evaluators’ past experiences and perceptions of an animal’s pain. A visual analogue scale would be difficult to apply in a busy private practice setting and would require additional training.
Intuitively, the smaller incision required for the LAOHE would seem to account for differences in pain with laparoscopic or laparoscopic-assisted procedures, compared with open procedures. Pain that occurs after an open procedure is also attributed to desiccation of exposed viscera and disruption of the peritoneal surface.²⁴ Less pain associated with LAOHE, compared with OHE, is also attributed to the relatively atraumatic nature of bipolar cauterization and transection of the ovarian pedicle, compared with digital disruption of the suspensory ligament. Digital disruption of the suspensory ligament extends to the peritoneal attachment and exposes retroperitoneal space. During this procedure, 9 of 10 patients in the OHE group had signs of pain. In contrast, similar signs of pain were not detected during bipolar cauterization and division.
Blood glucose and serum cortisol concentrations are useful measures of surgical stress. Blood glucose remained significantly increased for 6 hours after extubation in the OHE group and only for 1 hour in the LAOHE group. Additionally, significant increases from baseline of cortisol concentrations occurred only in the OHE group. Laparoscopic and thoracoscopic procedures or other effective measures of pain control reduce surgical stress.¹⁸,²⁵⁻²⁷ Other causes of increased glucose and cortisol concentrations include the stress of hospitalization. Our patient population consisted of shelter dogs acclimated to kennel confinement. To minimize variations associated with hospitalization, all patients were hospitalized 24 hours prior to entering the study.
It is well accepted that preemptive analgesia is important to limit the overall degree of pain a patient has after a given procedure.²⁸⁻³⁰ Despite the advances in recognition and management of pain that have occurred in veterinary medicine, inadequate management of pain is commonplace.³¹,³² Many dogs ovariohysterectomized in general practice are managed as outpatients, with minimal additional pain relief. Results of the present study illustrated that minimally invasive surgery can be viewed as a maneuver to reduce or preempt pain after surgery. One may consider minimally invasive methods more appropriate in an outpatient setting typical of general practice.
The benefits of veterinary laparoscopy or laparoscopic-assisted procedures have been validated by an enormous amount of clinical experience with laparoscopic surgery in humans. Yet, minimally invasive procedures have been slow to gain widespread use in small animal surgery. Laparoscopic-assisted ovariohysterectomy as described is less complex than laparoscopic OHE because of the use of 2 ports, minimal instrumentation, and conventional extracorporeal techniques. Results of our study provided evidence for reduced postoperative pain, whereas duration of surgery and anesthetic and surgical complications were similar.
Since completion of this study, the first author has modified the LAOHE technique to use 1 portal. A pneumoperitoneum is established, a 12-mm cannula is placed on midline at the level of the fourth mammary gland, and the operative laparoscope is inserted into the abdominal cavity. Retraction of the spleen and intestines is facilitated by rotation of the patient to right or left lateral recumbency, which allows the surgeon to see the abdominal viscera and identify the left or right ovariouterine complex, respectively. The ovaries are grasped and brought to the body wall, which allows percutaneous advancement of a transabdominal suspension suture; the suspensory ligament and ovarian vasculature are progressively cauterized and divided. After division of both ovarian pedicles, the patient is returned to dorsal recumbency. The proximal portion of the uterus is grasped with the multifunctional grasping forceps approximately 0.5 cm caudal to the ovary, the transabdominal suspension sutures are released, and the secured uterine horn is retracted into the 12-mm cannula. The cannula, laparoscope, bipolar graspers, and proximal portion of the uterus are retracted out of the portal for exteriorization of the uterine horn and associated ovary. The opposite uterine horn is exteriorized by a hand-over-hand maneuver, exposing the junction of the uterine body and uterine horn and subsequently the ovary. The uterine body is transfixed, ligated, and divided in an extracorporeal fashion. The single portal site is closed in 2 layers with absorbable monofilament and nylon suture. This modification allows further reduction in the impact of the surgical procedure by elimination of extra entries into the peritoneal cavity and reduces the number of endosurgical instruments required.
a. Remedios A, Ferguson J, Walker D, et al. Laparoscopic versus open ovariohysterectomy in dogs: a comparision of postoperative pain and morbidity (abstr). Vet Surg 1997;26:425.
b. Venocath-16, Abbott Ireland Ltd, Sligo, Ireland.
c. Accu-Chek Advantage, Roche Diagnostic Corp, Indianapolis, Ind.
d. Coat-A-Count, Antech Diagnostics, Farmingdale, NY.
e. Surgassist, Biovision Technologies, Golden, Colo.
f. Operating laparoscope (11 mm), Biovision Technologies, Golden, Colo.
g. PDS, Ethicon, Sommerville, NJ.
h. Endoblade, Biovision Technologies, Golden, Colo.
i. GraphPad Prism, version 4.00 for Macintosh, GraphPad Software, San Diego, Calif.
References
1. Pollari FL, Bonnett BN, Bamsey SC, et al. Postoperative complications of elective surgeries in dogs and cats determined by examining electronic and paper medical records. J Am Vet Med Assoc 1996;208:1882–1886.
2. Wallace MS. The ovarian remnant syndrome in the bitch and queen. Vet Clin North Am Small Anim Pract 1991;21:501–507.
3. Kyles AE, Douglass JP, Rottman JB. Pyelonephritis following inadvertent excision of the ureter during ovariohysterectomy in a bitch. Vet Rec 1996;139:471–472.
4. Coolman BR, Marretta SM, Dudley MB, et al. Partial colonic obstruction following ovariohysterectomy: a report of three cases. J Am Anim Hosp Assoc 1999;35:169–172.
5. Bradley KJ, Billet JP, Barr FJ. Dysuria resulting from an encapsulated haematoma in a recently spayed bitch. J Small Anim Pract 2000;41:465–467.
6. Merlo M, Lamb CR. Radiographic and ultrasonographic features of retained surgical sponge in eight dogs. Vet Radiol Ultrasound 2000;41:279–283.
7. Stocklin-Gautschi NM, Hassig M, Reichler IM, et al. The relationship of urinary incontinence to early spaying in bitches. J Reprod Fertil Suppl 2001;57:233–236.
8. Fingland R. Ovariohysterectomy. In: Bojrab M, ed. Current techniques in small animal surgery. 4th ed. Philadelphia: Lippincott Williams & Wilkins, 1998;489–493.
9. Austin B, Lanz OI, Hamilton SM, et al. Laparoscopic ovariohysterectomy in nine dogs. J Am Anim Hosp Assoc 2003;39:391–396.
10. Davidson EB, Moll HD, Payton ME. Comparison of laparoscopic ovariohysterectomy and ovariohysterectomy in dogs. Vet Surg 2004;33:62–69.
11. Van Goethem BE, Rosenveldt KW, Kirpensteijn J. Monopolar versus bipolar electrocoagulation in canine laparoscopic ovariectomy: a nonrandomized, prospective, clinical trial. Vet Surg 2003;32:464–470.
12. Bailey J, Freeman L. Endosurgery. In: Bojrab M, ed. Current techniques in small animal surgery. 4th ed. Philadelphia: Lippincott Williams & Wilkins, 1998;729–746.
13. Syrakos T, Antonitsis P, Zacharakis E, et al. Small-incision (mini-laparotomy) versus laparoscopic cholecystectomy: a retrospective study in a university hospital. Langenbecks Arch Surg 2004; 389:172–177.
14. Ros A, Gustafsson L, Krook H, et al. Laparoscopic cholecystectomy versus mini-laparotomy cholecystectomy: a prospective, randomized, single-blind study. Ann Surg 2001;234:741–749.
15. Alponat A, Cubukcu A, Gonullu N, et al. Is minisite cholecystectomy less traumatic? Prospective randomized study comparing minisite and conventional laparoscopic cholecystectomies. World J Surg 2002;26:1437–1440.
16. Bernstein MA, Dawson JW, Reissman P, et al. Is complete laparoscopic colectomy superior to laparoscopic assisted colectomy? Am Surg 1996;62:507–511.
17. Rawlings CA, Mahaffey MB, Bement S, et al. Prospective evaluation of laparoscopic-assisted gastropexy in dogs susceptible to gastric dilatation. J Am Vet Med Assoc 2002;221:1576–1581.
18. Walsh P, Remedios A, Ferguson J, et al. Thoracoscopic versus open partial pericardectomy in dogs: comparision of postoperative pain and morbidity. Vet Surg 1999;28:472–479.
19. Wright D, Serpell M, Baxter J, et al. Effect of extraperitoneal carbon dioxide insufflation on intraoperative blood gas and hemodynamic changes. Surg Endosc 1995;9:1169–1172.
20. Rosin D, Kuriansky J, Rosenthal RJ, et al. Laparoscopic transabdominal suspension sutures. Surg Endosc 2001;15:761–763.
21. Rodgerson DH, Belknap JK, Wilson DA. Laparoscopic ovariectomy using sequential electrocoagulation and sharp transection of the equine mesovarium. Vet Surg 2001;30:572–579.
22. Hannallah R, Broadman L, Belman A, et al. Comparison of caudal and ilioinguinal/iliohypogastric nerve blocks for control of post-orchiopexy pain in pediatric ambulatory surgery. Anesthesiology 1987;66:832–834.
23. Conzemius MG, Brockman DJ, King LG, et al. Analgesia in dogs after intercostal thoracotomy: a clinical trial comparing intravenous buprenorphine and interpleural bupivacaine. Vet Surg 1994; 23:291–298.
24. Watson RW, Redmond HP, McCarthy J, et al. Exposure of the peritoneal cavity to air regulates early inflammatory responses to surgery in a murine model. Br J Surg 1995;82:1060–1065.
25. Naitoh T, Garcia-Ruiz A, Vladisavljevic A, et al. Gastrointestinal transit and stress response after laparoscopic vs conventional distal pancreatectomy in the canine model. Surg Endosc 2002; 16:1627–1630.
26. Marcovich R, Williams AL, Seifman BD, et al. A canine model to assess the biochemical stress response to laparoscopic and open surgery. J Endourol 2001;15:1005–1008.
27. Fox SM, Mellor DJ, Firth EC, et al. Changes in plasma cortisol concentrations before, during and after analgesia, anaesthesia and anaesthesia plus ovariohysterectomy in bitches. Res Vet Sci 1994; 57:110–118.
28. Bailey J, Pablo L. Anesthetic and physiologic considerations for veterinary endosurgery. In: Freeman L, ed. Veterinary endosurgery. St Louis: CV Mosby Co, 1999;24–43.
29. Lascelles BD, Cripps PJ, Jones A, et al. Post-operative central hypersensitivity and pain: the pre-emptive value of pethidine for ovariohysterectomy. Pain 1997;73:461–471.
30. Lascelles BD, Cripps PJ, Jones A, et al. Efficacy and kinetics of carprofen, administered preoperatively or postoperatively, for the prevention of pain in dogs undergoing ovariohysterectomy. Vet Surg 1998;27:568–582.
31. Hellyer PW. Treatment of pain in dogs and cats. J Am Vet Med Assoc 2002;221:212–215.
32. Wagner AE, Hellyer PW. Survey of anesthesia techniques and concerns in private veterinary practice. J Am Vet Med Assoc 2000; 217:1652–1657.
From the Veterinary Surgical Services, Veterinary Referral Center of Colorado, 3550 S Jason St, Englewood, CO 80110 (Devitt); the Deer Creek Animal Hospital, 10148 W Chatfield Ave, Littleton, CO 80127 (Cox); and My Pet’s Place, 9111 S Santa Fe Dr, Littleton, CO 80125 (Hailey).
Supported by Biovision Corporation, Golden, Colo.
Address correspondence to Dr. Devitt.